Autophagy, also called “autolysis” or “autophagocytosis”, comes from the Greek word for “eating oneself”. It is a natural mechanism that consists of the partial degradation of the cell’s contents by the cell itself, cleaning, and recycling within the cell. This phenomenon has been known since the 1960s thanks to the medical doctor and biochemist Christian de Duve. Nevertheless, Professor Yoshinori Ohsumi received the Nobel Prize in Medicine and Physiology in 2016 for his discoveries on the mechanisms of autophagy.
This technical but necessary article to understand the benefits of Autophagy is intended for the most curious. We have tried to make this article as explicit as possible so that you can understand all the mechanisms of this precious phenomenon: Autophagy.
Short preamble
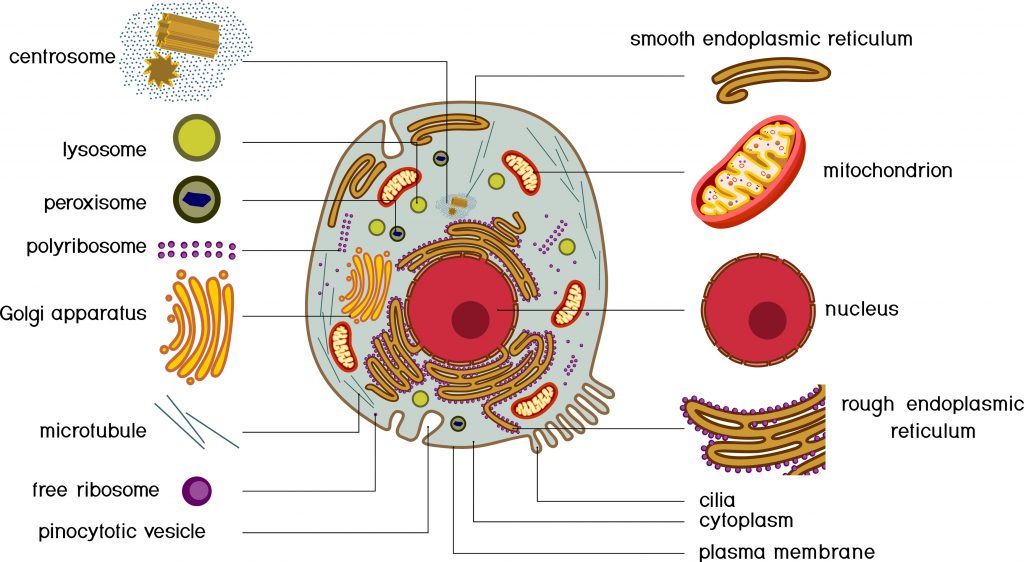
Cytoplasm: It indicates all the space located between the nucleus and the plasma membrane. It consists of cytosols and organelles.
Cytosol: The semi-liquid fraction of the cytoplasm.
Cellular organelle: Specialized structure contained in the cytoplasm and delimited from the rest of the cell by a phospholipid membrane. There are many types of organisms in eukaryotic cells, the main ones being: the nucleus, the endoplasmic reticulum, the golgi apparatus, the mitochondria, the lysosomes, the peroxisomes, etc.
Nucleus: Cellular organelle that contains most of the cell’s genetic material, as well as the machinery necessary for the replication of chromosomes and the expression of the information contained in the genes. It is surrounded by a double membrane called the nuclear envelope.
Lysosome: Cellular organelle present in the cytosol whose function is to carry out intracellular digestion thanks to about forty enzymes.
Ribosome: Cellular organelle synthesizes the proteins that make up each of our cells by decoding the information in the messenger RNA.
What is autophagy?
Autophagy is a process of self-degradation of cell components designed to regulate various cellular functions. These functions are : growth, differentiation, response to starvation or oxidative stress, programmed cell death, and renewal of damaged intracellular components. In addition, it plays a role in the maintenance and cleaning of damaged organelles, misfolded or aggregated proteins, and invasive pathogens inside the cell.
Authophagy is also a recycling mechanism. Indeed, the sub-products of degradation will be reused to produce new building blocks and energy. Thus, in response to deprivation of nutrients, amino acids, growth factors, or oxygen, autophagy is induced to provide another energy source to cells from recycled cellular material to help them survive. It is therefore considered as a key component of the adaptive response of cells and organisms to stress. Indeed, it promotes survival until nutrients become available again. In addition, it helps maintain the integrity of the cell by renewing it and ensuring homeostasis in general.
Molecular characteristics & mechanisms
Historically, autophagy was considered to be a non-selective process of degradation of parts of the cytoplasm during conditions of starvation or low energy levels within the cell, to promote cell survival by recycling cytoplasmic material. But there is growing evidence for selective autophagy to degrade damaged organelles, aggregated proteins, and pathogenic microorganisms. The process is carried out by different proteins called ATG (Autophagy-Related Gene).
There are 3 types of autophagy:
- macro-autophagy
- micro-autophagy
- chaperone-mediated autophagy (CMA)
When autophagy is mentioned, it usually refers to macro-autophagy.
Macro-autophagy
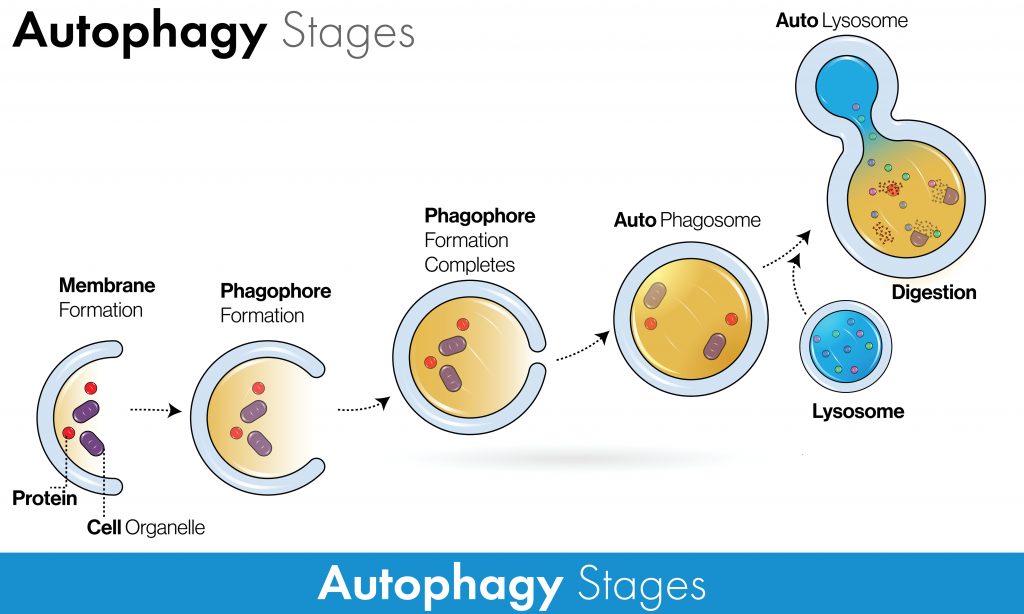
In macro-autophagy, there are several key steps:
- An isolation membrane called a “phagophore” begins to form around the selected or unselected components.
- The edges of the phagophore expand and close, forming a new structure known as an “autophagosome.”
- The closed, component-laden autophagosome will merge with the “lysosome”, the cell’s recycling factory, to form an “autolysosome”. It contains enzymes known as lysosomal hydrolases that digest and degrade the contents.
- This process generates amino acids and other degradation by-products that can be recycled and reused for construction.
Micro-autophagy
In micro-autophagy, selected or unselected components are directly taken up by the lysosome itself by folding the lysosomal membrane.
Chaperone-mediated autophagy
In chaperone-mediated autophagy, selected components are moved through the lysosome in a complex with chaperone proteins resulting in their expansion and degradation.
When is autophagy triggered?
Constantly, our cells keep both selective and non-selective autophagy processes at low levels: it is called “basic autophagy”. However, non-selective autophagy is significantly triggered when the organism faces various stresses and physiological conditions. These conditions can be: fasting, hyperthermia, or hypoxia. Although selective autophagy can also be induced in response to stress: it is called “significant autophagy”.
The main products of autophagy are amino acids derived from cellular proteins. The onset of autophagy is very rapid and occurs before energy fuels are completely exhausted. For example, 24-hour starved mice show increased autophagy in many tissues but still have sufficient lipids (glycogen can be consumed on the first day). Therefore, it is unlikely that autophagy provides energy in these settings. Indeed, several studies have suggested that autophagy-derived amino acids are used to synthesize proteins essential for adapting to starvation. Cellular restoration of amino acid levels reactivates the serine/threonine protein kinase mTORC1 and terminates autophagy.
Fasting can increase autophagy levels
The central question is whether the observed benefits are related to this increase in autophagy or whether it is simply a correlation. Thus, in the absence of clear answers, the mistake we regularly make is to try to fast long enough to know how long to fast to induce significant autophagy. However, let’s remember that autophagy has several roles. On the one hand, the selective degradation of damaged components to guarantee the integrity of the cell permanently. And on the other hand, the non-selective degradation of a portion of the cytoplasm to allow the cell to adapt and survive in the face of stress while waiting for the return to normal.
The stress response induces, above all, non-selective autophagy for adaptation and cell survival. We conclude, contrary to what is frequently argued by fasting specialists, that one should not seek to fast to induce significant (mainly non-selective) autophagy. But, one should fast to guard against any impairment of basic (mainly selective) autophagy over time and occurs when the cell is fed. Indeed, a deficiency of the so-called basic selective autophagy leads to many pathologies.
Differentiating autophagy, apoptosis, and necrosis
Cell death is fundamental for the proper execution of normal body processes. However, excessive cell death is involved in developing pathologies such as AIDS and neurodegenerative diseases. On the other hand, the capacity of cells to die favors the emergence of cancers. There are 3 forms of cell death: autophagy, apoptosis, and necrosis.
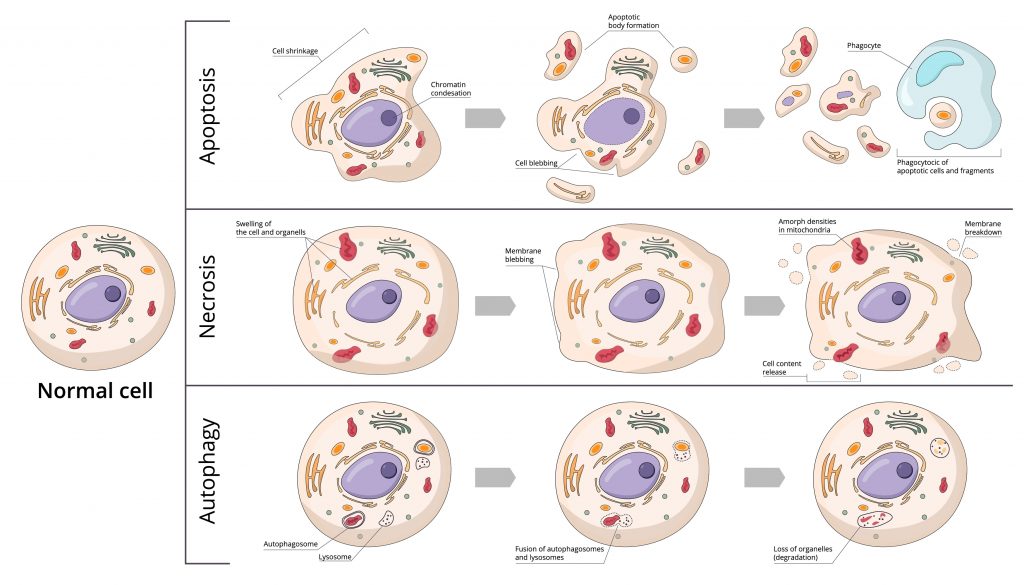
Autophagy
It is a degradation and cleaning process where cells destroy their components via the lysosomal machinery and a recycling process. While apoptosis fulfills its role by dismantling damaged or unwanted cells, autophagy maintains cellular homeostasis through the selective recycling of intracellular organelles. Unlike apoptosis, autophagy has little or no association with phagocytes.
Yet, under certain conditions, autophagy can sometimes go as far as cell death when intracellular damage is too significant, and apoptosis is compromised. The same constraints can stimulate apoptosis and autophagy. It is now known that there are exchanges between the apoptotic and autophagic pathways, and studies have shown that BCL2, an important regulatory gene for apoptosis, is also an important regulator of autophagy.
Apotosis
Apoptosis is a programmed cell death directed by genes. Apoptosis is involved in the elimination of incompetent or useless immune cells, as well as the elimination of cells with irreparable DNA damage. It has two components: the first phase is the initiation of cell death, and the second phase is the elimination of the dying cell. The elimination of the dying cell usually begins before the cell and is completely dismantled via specialized phagocytosis. It is, therefore a normal and necessary phenomenon for the survival of multicellular organisms. It is in constant balance with cell proliferation.
Deregulation of apoptosis can be the origin of many pathologies. Some are linked to an inhibition of apoptosis (cancer, lymphoproliferative syndromes, etc.), while others are associated with stimulation of this phenomenon (AIDS, neurodegenerative disease, autoimmune disease, etc.). Apoptosis is accompanied by a reduction in cell volume, DNA fragmentation, bleeding from the plasma membrane, and engulfment by phagocytes.
Necrosis
It is considered to be a passive, unscheduled form of cell death. Necrosis can be caused by a decrease in blood supply causing hypoxia long enough to asphyxiate tissues, trauma altering blood circulation, bacterial and/or possibly fungal infection, cancerous process, or venoms. Necrosis is, therefore, a pathological phenomenon. However, recent data suggest that it may also represent a form of programmed cell death whose activation may have important consequences, such as inducing an inflammatory response. It is accompanied by increased cell volume, swelling of organelles, rupture of the plasma membrane, and loss of intracellular content.
Autophagy in health and disease
Basic autophagy allows, as we have seen, the selective cleaning of damaged intracellular components or invasive pathogens. Therefore, its action under normal conditions protects the cell against DNA damage, which could otherwise lead to tumor formation. It is considered to be a cancer-suppressing factor.
Not surprisingly, data show that this basic level of autophagy deficiency is associated with tumorigenesis (tumor formation) and neurodegenerative disorders (Alzheimer’s, Parkinson’s, liver disorders, amyotrophic lateral sclerosis).
On the other hand, high baseline autophagy is observed in several types of cancers, such as pancreatic cancers. In treating these cancers, inhibition of elevated autophagy by drug treatments decreases cell proliferation and promotes tumor suppression. In cancer biology, autophagy is thus considered to play a dual role in tumor suppression but also tumor development.
“Autophagy plays a dual role in tumor suppression and development.”
Indeed, several studies indicate that autophagy promotes tumor survival and growth in advanced cancers. When tumors are exposed to extremely stressful conditions, including hypoxia and nutrient deprivation, autophagy helps cells overcome these stresses and obtain the large amounts of energy they need to promote their survival. Autophagy is thought to function as a tumor suppressor in non-tumor cells or in the early stages of tumor cell development. Still, conversely, it is thought to promote the survival of cancer cells once tumors are established.
Bottom line
So finally: is it rather unfavorable to fast to induce autophagy when one already has cancer? The answer cannot be based only on the autophagic process because during a fast, there is a diversity of other processes that occur parallel to the rise of non-selective autophagy. In practice, we see cancer patients experimenting with long fasts and getting miraculous and sometimes catastrophic results.
Therefore, it cannot be said that prolonged fasting (over 20 days) is the most appropriate solution for advanced cancer. On the one hand, the significant autophagy induced will also allow cancer and non-cancerous cells to adapt and survive. On the other hand, if the other processes induced during long fasting do not sufficiently degrade the vast majority of the tumor, then the increase in growth factors that takes place during the resumption of feeding. And, the increase in our sensitivity to these factors (all the more important after a long fast) will feed the remaining cancer cells and favor their development. Thus, for advanced cancers, we could assume that it would be more prudent and interesting to opt for a ketogenic diet that does not induce significant autophagy and to do regular short fasts (dry fasts included).
If you want to learn more about fasting, don’t hesitate to have a look on my dedicated website: Jeûne & Sens
Interested in fasting? Have a look at our other fasting articles: